Introduction:
Chimeric antigen receptor (CAR) T-cell therapy is emerging as a novel and revolutionary therapy in the treatment of relapsed or refractory lymphoma and other hematological malignancies. Patients with hematological malignancies are at risk of bleeding and thrombosis due to multiple risks factors. As there is an ongoing exploration of the incidence and characteristics of bleeding and thrombosis after CAR T-cell therapy, there is no existing published data that incorporates both peer-reviewed and non-peer-reviewed literature.
Aim:
Assess the risk of bleeding and thrombosis in CAR T-cell recipients for hematological malignancies.
Method:
We conducted a systematic literature search in MEDLINE, EMBASE, and CENTRAL electronic databases from inception to October 2022 using a combination of subject headings and text words. We included studies that assessed thrombotic and bleeding complications in patients eighteen or older who underwent CAR T-cell therapy for a hematologic malignancy. Our search strategy yielded 14 studies in the form of abstracts and full-text articles. The review process consisted of two levels of screening: (1) a title and abstract review and (2) a full-text review.
Result:
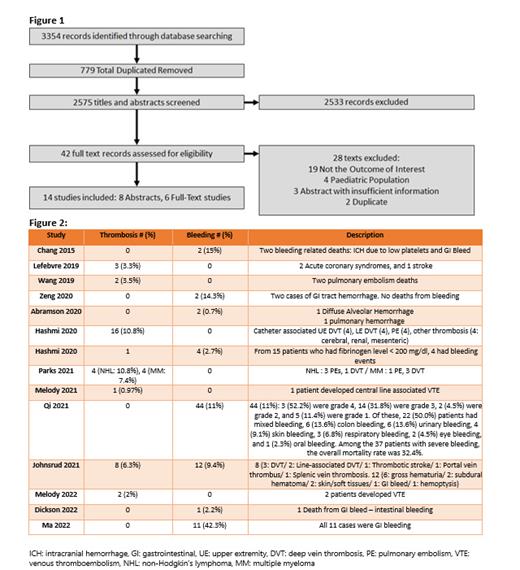
Of 3354 records, we included 14 studies based on our inclusion criteria [figure 1]. Majorities of the studies were retrospective. Most of the patients in these studies received CAR T-cell therapy for refractory diffuse large B-cell lymphoma. Patients received at least 2 lines of therapy or more before CAR T-cell treatment. Different types of CAR T-cell therapy were used. Based on the included studies, the risk of bleeding was higher than thrombosis [figure 2]. Bleeding sites were variable, but gastrointestinal (GI) bleeding was predominant. In Ma et al, GI bleeding occurred in 11 (42.3%) patients. In Qi et al and Johnsrud et al, bleeding occurred in 44 (11%) and 12 (9.4%) patients respectively. Variable sites of thrombosis were noticed. In Hashmi et al incidence of thrombosis was 10.8%. Although there are some risk factors associated with bleeding and thrombosis in this population, the exact mechanism is still unknown. We think future studies to determine the exact mechanism of this toxicity and to assess the underlying risk factors would be crucial to decrease morbidity and mortality in this population and to develop risk assessment tools.
Disclosures
No relevant conflicts of interest to declare.